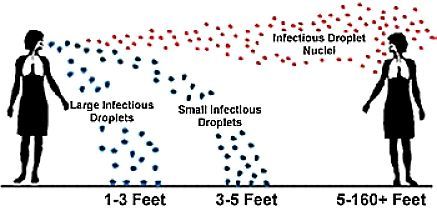
Since the previous post concerning the circulating 2019-nCoV, researchers have been busy trying to answer some of the many unknowns about this novel virus. As of January 31st, 2020 the US declared a public health emergency, with a total of 12 confirmed cases. However, the fear lies in the human-to-human transmission that is suspected to occur through respiratory droplets. This form of transmission means that a healthy person must somehow transfer a droplet containing virions from an infected person to the appropriate portal of entry, through the respiratory tract, either by inhaling the droplets from a nearby contagious person (1-5 ft range in the diagram above) or moving them with the hands. The estimated R-naught value for 2019-nCoV is 2.2, indicating the average number of people that will contract the disease from one contagious person. This number indicates that close contact is necessary, and that prevention is possible with better hand-washing and mouth covering technique.
From this report, it appears that the virus can be spread during its incubation period as several German workers were passed the disease by an asymptomatic Chinese business contact. This type of evidence is what pushes the US to take great precaution, and continue the declaration of a public health emergency even with a low number of confirmed cases. Because the incubation period is still unknown, with an estimated range of 2-14 days, it will be difficult to identify those infected and potentially spreading the disease to their contacts. That same report from Germany also found a high viral content in the sputum of one patient during the convalescent period. Therefore, even when an infected person is in the recovery phase, transmission is still possible.
With the death toll climbing in China, now at 636, the investigation into antiviral drugs is heightening. This article displays the effectiveness, in vitro, of Remdesivir and Chloroquine. Remdesivir has shown promise against an array of RNA viruses, by incorporating into nascent viral RNA chains and causing early termination. Chloroquine, used commonly as an anti-malarial and autoimmune disease drug, and works by increasing the endosomal pH required for cell-virus fusion, and interfering with glycosylation of host cellular receptors for the virus. Since both of these have already gone through appropriate safety testing, they would be easy options for testing against the 2019-nCoV. Some HIV protease inhibitors are also being considered, since the 2019-nCoV’s viral protease is noted to be essential for its replication. This kind of research is crucial in halting the death toll, whether the rate of transmission subsides or not.

The question of the passage of transmission, mentioned in the previous post, of this originally zoonotic disease in the Wuhan market has also been further investigated. Although the work is not published, researchers have recovered coronaviruses with 99% genetic similarity to the circulating 2019-nCoV from the pangolin (pictured above). The illegal trading of this animal is widespread. Therefore, even though it is not listed as a product at the Wuhan market, its presence there is possible. Further investigation is needed to confirm this observation. If identification is confirmed, at least one end of the transmission loop can be closed.
