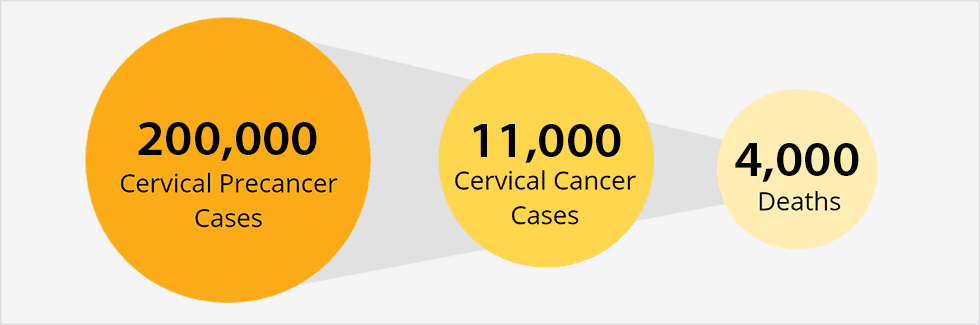
There are only two cancer preventing vaccines currently available to the American public: the hepatitis B virus (HBV) vaccine and the human papillomavrius (HPV) vaccine. HPV’s are nonenveloped double stranded DNA viruses spread through intimate skin-to-skin contact. Many serotypes (~150) have been identified, and key strains are heavily linked to the development of cervical, anogenital, oropharyngeal, head, and neck cancers. Low-risk strains, such as types 6 and 11, cause low grade cervical cell changes and genital warts. High-risk strains, including types 16 and 18, encode several oncoproteins in their 8 Kb circular genome that manipulate cell cycle regulators, induce chromosomal abnormalities, and block apoptosis. These processes result in the continued abnormal differentiation of epithelial cells that can easily lead to tumors. The integration of the viral genome into the host genome shields the infection from the host’s immune response. There is no treatment for HPV infections besides removal of local legions, which does not usually eliminate infection.
Despite the suggestion that essentially all cervical cancers are attributable to HPV, under 50% of the appropriate age groups are properly vaccinated. When the previously linked article was published, only 2 vaccines were used in the U.S: Cervarix, a bivalent for types 16 and 18; and Gardisil, a quadrivalent for types 6, 11, 16, and 18. Gardisil 9 (9vHPV) has since been introduced, which covers 5 additional cancer-causing serotypes and is now the only HPV vaccine in used in America. 9vHPV is a noninfectious virus-like particle (VLP) vaccination which underwent thorough clinical trials in over 15,000 females and males aged 9-26. All adverse effects were reported, most commonly relating to non-severe injection site events including pain, swelling and erythema with mild-to-moderate intensity. These events were reported more frequently than for the two previously used vaccines, but the protection it confers is deemed much more significant than short-lived irritation at the injection site.
Even with vaccine safety confirmed, vaccine refusal and misinformation are very common. Two doses of the HPV vaccine are recommended for all boys and girls at ages 11-12. If the sequence is started after age 15, 3 doses over 6 months, is recommended. In 2017, coverage among boys was 44.3%, compared with 53.1% in girls. Neither percentage is impressive, and the apparent gender gap relates to physician recommendations and misinformation among parents and teens. In the JAMA Pediatrics research letter, polling revealed that 60.1% of men, and 31.6% of women did not know HPV caused cervical cancer. Additionally, over 75% of adults were unaware that the virus also contributes largely to incidences of anal, oral, and penile cancers. A recent Pediatrics study investigated the recommendation practices of pediatricians and family physicians (FPs). The researchers emphasized the importance of using the “presumptive” style when discussing the vaccine, meaning it was introduced as routine along with menigitis and DTaP/TDaP vaccinations. Only 65% of pediatricians and 42% of FPs used this approach always or almost always. Ample room for improvement was identified in both stylistic approaches to introducing the vaccine to families, and emphasis on covering both boys and girls aged 11-12. It was noted that the 2 dose sequence was much more likely to be completed than the 3 dose sequence after age 15. Therefore, early introduction and adherence is crucial in eliminating HPV induced cancers from our nation’s medical concerns.