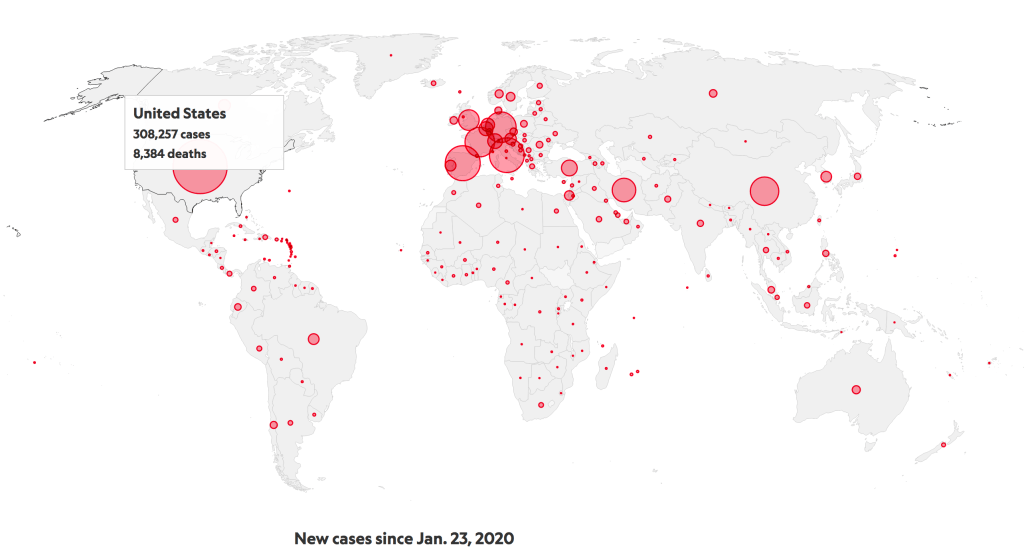
In my last post about COVID-19, I said the U.S. was “past the stage of true containment.” That was on March 7, when North Carolina had about 7 confirmed cases. There are now about 2600 reported cases, but I hope the public realizes that this count is lagging, inaccurate, and anything but a true representation of disease spread in the state. Unfortunately, these assertions also apply to the nationwide total that now surpasses 300,000. Lack of testing and asymptomatic carriers are the main inhibitors of accurate reporting. The panic and anxiousness of the world could subside if one of the current drug or vaccine trials showed promise in reducing COVID-19 associated deaths. Even with the hundreds of clinical trials occurring, with a demographic of some of the sickest coronavirus patients, a overwhelmingly positive treatment has failed to arise. The following post will explore the potential of Remdesivir, an intravenous drug that interrupts viral protein synthesis, and one vaccine candidate, Fusogenix DNA vaccine by Entos Pharmaceuticals.
The current clinical trials for Remdesivir against COVID-19 being carried out in the United States can be viewed here. Of the four, three are sponsored by Gilead Sciences, as they announce their donation of 1.5 million doses of the experimental drug, and one study is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). The latter had an estimated completion date of April 1st, but no results have been posted. Two of the Gilead studies are very similar besides one being classified as enrolling patients with “severe” coronavirus disease versus “moderate” coronavirus disease. Both cohorts were required to be hospitalized in order to qualify for the study, but their condition was distinguished by whether peripheral capillary oxygen saturation was above or below 94%. The third Gilead study required patients to be currently requiring mechanical ventilation upon enrollment. The NIAID sponsored study required some type of severe lung involvement. Hopefully this range of cohorts and dosage plans can reveal if Remdesivir is able to reduce viral load and mortality for COVID-19. This article, in the International Journal of Antimicrobial Agents, purports the broad-spectrum anti-coronavirus activity of Remdesivir in both in vitro and in vivo animal experiments. Its ability to be metabolized into a nucleoside analogue tricks the virus’s RNA dependent RNA polymerase into incorporating the drug into viral proteins, which are then terminated prematurely when the next nucleoside is unable to be added. If the virus cannot make effector and structural proteins, it dies, giving the host a break from its torture. The aforementioned studies will determine whether the drug can migrate to the location of the infection and appropriately enter human tissue cells where the damage is occurring.
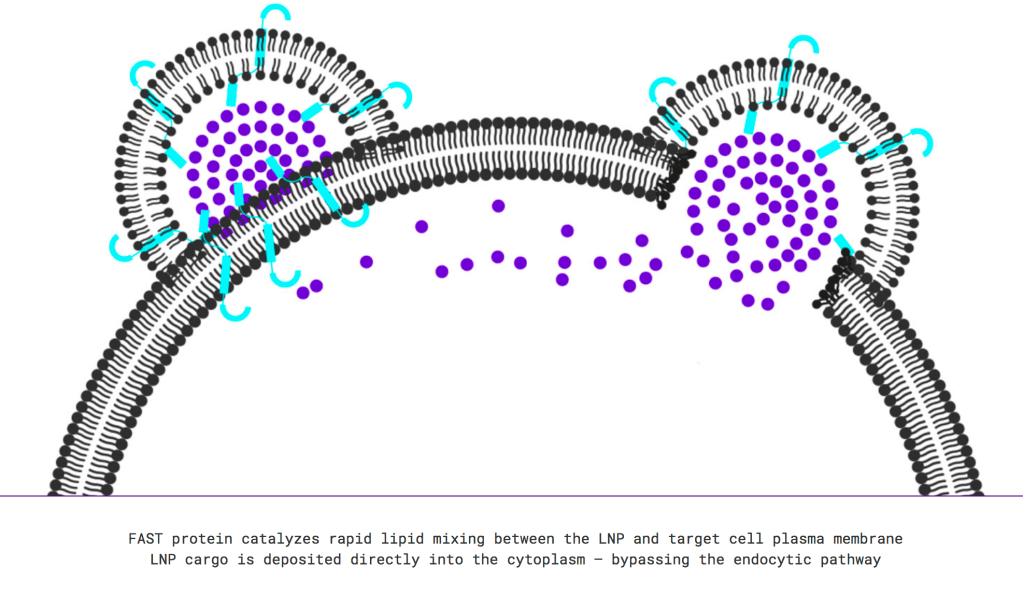
Creating and making a vaccine against COVID-19 is a much longer road than evaluating the efficacy of an FDA approved drug to fight the infection. Convincing basic science, animal trials, and long term human trials are all necessary parts of vaccine development. A more extensive list of vaccine candidates can be found here. The Fusogenix DNA vaccine by Entos Pharmaceuticals uses a relatively newfound vaccine platform that delivers genetic payload directly to cells in the form of a plasmid. According to Entos representatives, once the formulation of the COVID-19 vaccine has been completed, a fast-paced route to human clinical trials is planned to bring the vaccine to the public. This optimistic assertion of an efficacious vaccine is based on the idea that the plasmid would be translated inside cells, producing non-dangerous antigens selected from this novel coronavirus, inducing an immune response from B and T cells. Other advantages include enhanced vaccine stability and the avoidance of infectious agents in the production of the vaccine. Because multiple proteins/antigens could be selected to be delivered via the lipidnanoparticle vesicle, there are endless optimization and modification options should the virus mutate.
In attempt to avoid the depression that comes with coronavirus daily news updates, I am trying to instead focus my searches on these drug and vaccine trials. The results of clinical trials cannot be published soon enough. Although time is moving slowly for the world under stay-at-home orders, we should take the time to appreciate the fast tracked science occurring in response to this global pandemic. Scientific capabilities grow everyday, and I am opportunistic that an answer will surface soon. Meanwhile, keep doing your part to slow the spread of this threatening virus.
