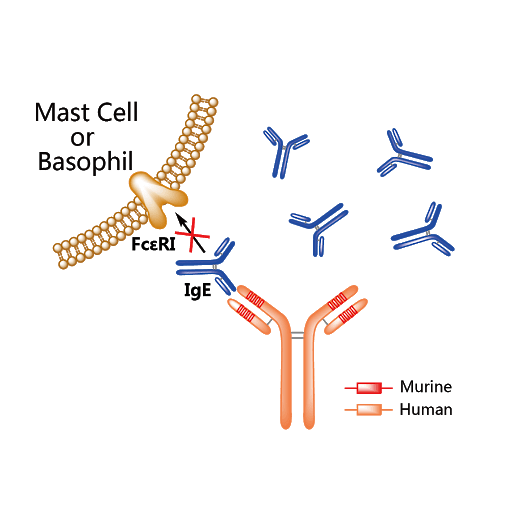Monoclonal antibody medications (usually ending in “mab”) are created to specifically target human physiology to reduce disease burden in a variety of afflictions, including certain cancers, rheumatoid arthritis, and asthma. Antibodies are produced naturally by our immune cells to neutralize potentially harmful foreign particles. Although these molecules are an essential part of our adaptive immune response, the B cells that produce them can be wrongly activated in some cases, producing self-damaging autoantibodies. Additionally, Ig-E class antibodies can mediate life-threatening allergic responses to non-pathogenic foreign substances. Scientists can modify the mouse immune system to produce human antibodies in response to a desired antigen, creating a pathway by which to harvest neutralizing agents that can be administered to patients. The term monoclonal indicates that every antibody produced came from cloned immune cells, and therefore only binds to one unique antigen.
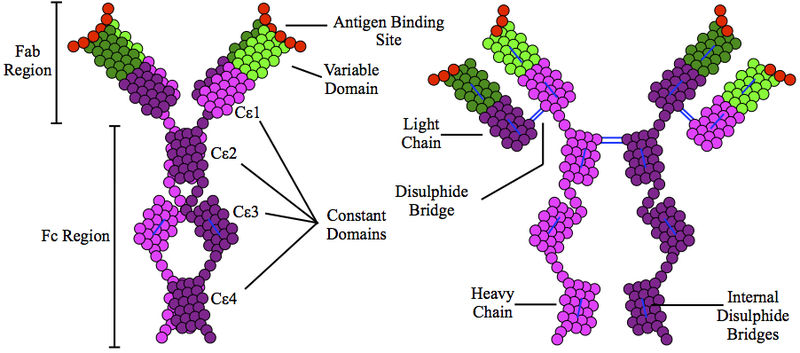
Omalizumab (Xolair) is an injectable monoclonal antibody drug used to treat severe cases of asthma and sometimes chronic idiopathic urticaria (hives), administered every 2-4 weeks by a healthcare provider. Individuals who are IgE sensitized against an allergen have IgE antibodies coating their mast cells and basophils. When the allergen enters their system, it crosslinks these IgE antibodies inducing degranulation of mast cells and basophils, which release histamine, cytokines, and other pro-inflammaotry molecules that can induce an allergic response ranging from mild hives to severe anaphylaxis. For people with asthma, IgE-mediated allergic responses can trigger asthma attacks. Omalizumab targets the IgE Fc region, acting as an anti-antibody. This artificial human antibody acts to reduce cell surface bound IgE, and was also found to reduce the number of FcεRI (high-affinity IgE Fc) receptors on basophils over time. By modulating a person’s acquired immune response against allergens, this drug can reduce asthma attacks in people with persistent asthma that is not well controlled by inhaled corticosteroids.
A forced interaction with IgE does not come without risk. Below are the side effects listed by the FDA for Omalizumab.
The most serious side effects:
- Anaphylaxis
- malignant neoplasms (cancer)
- inflammation of blood vessels
- fever
- muscle aches
- rash
- parasitic infection
- heart and circulation problems
The most common side effects:
- pain in arms or legs
- dizziness
- feeling tired
- bone fractures
- common cold symptoms
- nausea, vomiting
- nose bleeds
- joint pain
- upper respiratory tract infection
These possible adverse reactions are gathered from clinical trials data and are compared with instances of the same reactions/conditions in control groups to test whether there is a significant increase when the drug is present. For example, one study found malignant neoplasms in 0.5% of Omalizumab-treated individuals versus 0.2% of those receiving a placebo. With many confounding factors contributing to cancer, it can be difficult to determine whether the drug induced this difference or not. Either way, it has to be listed with other side effects. The drug manufacturer repeatedly highlights anaphylaxis as the most serious and noteworthy potential reaction, with an estimated 0.2% frequency post-administration. Because the drug is targeting mast cells and basophils’s degranulation pathways, which are responsible for the release of mediators that induce anaphylaxis, it is not surprising that degranulation could be accidentally activated in some individuals when FcεRI and IgE are kept from performing their usual roles.
The listing of heightened risk of parasitic infection is also interesting. Adaptive IgE responses are initiated by B cells that were activated by TH2 (T helper cell 2) cells. In a historical sense, this pathway is normally activated in response to helminth (worm) infections. In the developed world, where worms are uncommon, there has been an increase in IgE-mediated allergies. It is suspected that idle TH2 cells can contribute to the development of allergies in susceptible individuals. The ability of Omalizumab to downregulate binding of IgE to mast cells and basophils reduces the immune systems capacity to fight helminth infections. While this may not pose serious threats for people living in the US, it is a dangerous susceptibility to travelers and those living in underdeveloped nations. Still, it is remarkable that a drug so specific has been created for human therapy. Monoclonal antibody medications serve as yet another example of how mimicking nature ushers in some of the most successful scientific discoveries. Cost is an adverse factor, with one dose marketing for around $1200. Fortunately, other companies are hoping to introduce a biosimilar to Omalizumab which could reduce cost burden and increase treatment availability.